Our study coordinators and doctors are excited and motivated about finding a cure for epilepsy.
About Clinical Research and the Human Epilepsy Project
Our study coordinators and doctors are excited and motivated about finding a cure for epilepsy.
We want our participants to have a clear understanding about the HEP research study before they decide to participate. Patients under no circumstances will be forced to participate in the study and can change their mind at any time.
A patient during a yearly visit with DR manu HEgde M.D., Ph.D.
Patient-centered research
When a participant enrolls in the HEP study, they are matched with a study coordinator and a study doctor, who become that patient's Research Team. The study team will monitor the patient's epilepsy for at least two years and can often be a resource to help participants navigate the world of epilepsy care.
About Participating in the Human Epilepsy Project
The cohort for newly diagnosed participants is finished recruiting new participants, but HEP is recruiting participants with diagnosed idiopathic generalized epilepsy.
If you answer yes to the three questions below, you may be eligible to participate in HEP3:
Have you had at least 1 (absence, myoclonic, or generalized tonic-clonic convulsion) seizure in the 6 months prior to treatment?
Have you had AED treatment (for seizures) instituted no more than 12 months ago?
Are you 13 years old or older?
For assistance with deciding if you are eligible, contact us to express an interest in participating in HEP.
What will I need to do if I participate?
HEP 3:
HEP participants with diagnosed idiopathic generalized epilepsy will be followed up to two years. Participating takes a few hours at the baseline visit and then about an hour at each follow up visit. The big study visit is at enrollment where participants will answer questions about their epilepsy, take some online tests, give blood, urine, and stool samples, and track their seizures and medications with an electronic or paper seizure diary. Participants will also use a Fitbit Inspire HR to collect health features and sleep data. After the initial visit there are up to two consecutive annual check ups performed at the end of each year.
A patient is being examined by DR manu HEgde M.D., Ph.D. at UCSF San francisco, CA during a yearly visit
What is a medical research study like the Human Epilepsy Project?
Medical research is conducted to aid the body of knowledge in the field of medicine. This can be divided into two general categories: new treatments that are tested in clinical trials, and all other research contributing to the development of new treatments. HEP is a biomarkers research study, and there is no treatment that is being tested. Therefore, we are not a clinical trial. HEP investigators will be looking for patterns in DNA, proteins, MRI, and EEG, and other biomarkers that explain the development of focal epilepsy.
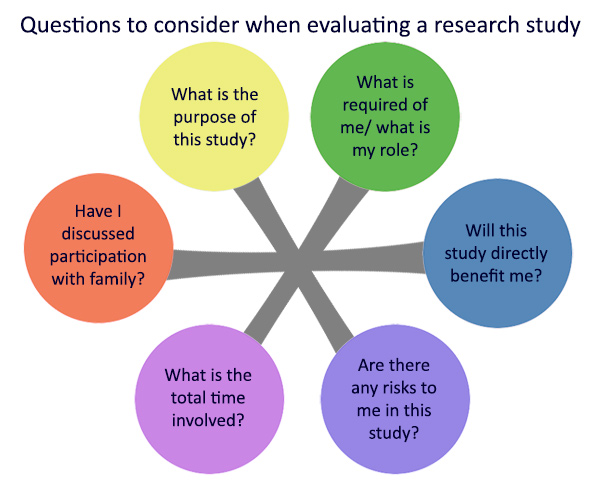
Here are some general questions to think about when you consider joining HEP:
The decision to participate in medical research is a very personal one. However, there are guidelines to protect study participants. Research in humans is permitted only in volunteers who have been briefed about both the potential benefits and hazards of trial participation. This is called informed consent. It is the responsibility of the research team to explain the risks to potential study participants.
For more information
Understanding Clinical Trials - An Introduction to Clinical Trials provided by the National Institutes of Health.